
correlation gave slightly different critical properties, the z factors from those two methods are the same. 2 in Real gases) gives z = 0.745.Įven though the Sutton correlation and the Piper et al. The details of the calculations are found in Table 2.įinally, looking up the z-factor chart (Fig. 2 in Real gases, we obtain z g = 0.745.ģ. From Sutton’s gas gravity method, γ g = 0.705 then, we obtain from Eq. Using Kay’s rules, we obtain from the known gas composition:Ģ. Where the molecular weight of air, M a, is 28.967. Because c r = c g p pc,Ĭalculating the relative density (specific gravity)Ĭalculate the relative density (specific gravity) of natural gas with the following composition (all compositions are in mol%):įirst, calculate the apparent mole weight from the information presented in Table 1. 1-2 in Isothermal compressibility of gases for the previously calculated values of p r = 3.200 and T r = 1.500 to give c r T r = 0.5. The compressibility is determined by first reading Figs. Next, the ratio of μ g/ μ ga is read from Fig.

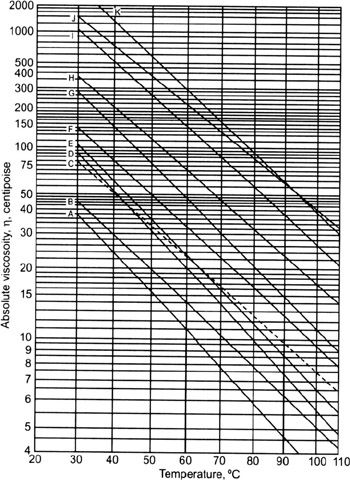
The correction for 10% CO 2 is 0.0005 cp, and the correction for 7% H 2S is 0.0002 cp. This gives 0.0102 cp, but corrections are needed for the acid gases.First, the viscosity for M g = (0.7)(28.967) = 20.3 at p = 1 atm and T = 75☏ is read from Fig.The viscosity is determined using the charts of Carr et al. 2 in Gas formation volume factor and density: The formation volume factor is calculated from Eq. 3 in Gas formation volume factor and density: 4 Calculating the z factor for a reservoir fluidįind the density, formation volume factor (FVF), viscosity, and isothermal compressibility of a gas with the following properties and conditions:.2 Calculating the relative density (specific gravity).1 Calculating properties of natural gas.


 0 kommentar(er)
0 kommentar(er)
